
ULTRAVATE (halobetasol propionate) 0.05% Lotion is a corticosteroid indicated for the topical treatment of plaque psoriasis in patients 18 years of age and older.
Baseline
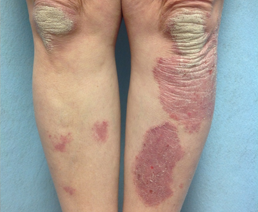
At Day 14
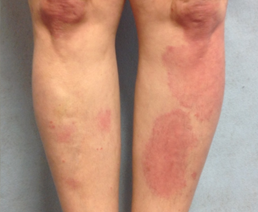
Female with plaque psoriasis on her lower leg, treated with ULTRAVATE Lotion twice daily for 14 days.*
*Individual patient results. Actual results may vary.
• |
ULTRAVATE (halobetasol propionate) Lotion is a corticosteroid indicated for the topical treatment of plaque psoriasis in patients 18 years of age and older. |
• |
Effects on Endocrine System: Reversible hypothalamic-pituitary-adrenal (HPA) axis suppression may occur, with the potential for glucocorticosteroid insufficiency during or after treatment. Systemic efforts of topical corticosteroids may also include Cushing's syndrome, hyperglycemia, and glucosuria. System absorption may require evaluation for HPA axis suppression. Use of potent corticosteroids on large areas, for prolonged durations, under occlusive dressings, or an altered skin barrier may increase systemic exposure. Children may be more susceptible to systemic toxicity when treated with topical steroids. |
• |
Local Adverse Reactions: Local adverse reactions with topical steroids may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection and miliaria. These may be more likely to occur with occlusive use, prolonged use or more potent corticosteroids, including ULTRAVATE. |
• |
Concomitant Skin Infections: Use an appropriate antimicrobial agent if a skin infection is present or develops. |
• |
Allergic Contact Dermatitis: Discontinue ULTRAVATE Lotion if allergic contact dermatitis is established. |
• |
In clinical trials the most common adverse reactions (≥1%) were telangiectasia, application site atrophy, and headache. |
References: 1. National Psoriasis Foundation. Topical steroids potency chart. https://www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart. Updated 2016. Accessed February 4, 2019. 2. Grove G, Zerweck C, Houser T, et al. Occlusivity and moisturization potential of a new lotion formulation of halobetasol propionate, 0.05%. Poster presented at: 74th Annual Meeting of the American Academy of Dermatology; March 4-8, 2016; Washington, DC. 3. ULTRAVATE® Lotion [prescribing information]. Cranbury, NJ: Sun Pharmaceutical Industries, Inc.; 2017.