
TOPICORT (desoximetasone) 0.25% Topical Spray is a corticosteroid indicated for the treatment of plaque psoriasis in patients 18 years of age or older.
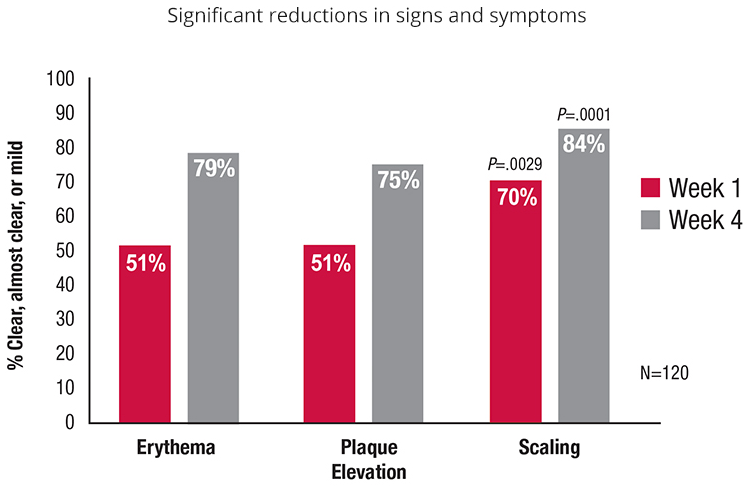
| * | The efficacy and safety of TOPICORT Topical Spray, 0.25% was evaluated for the treatment of moderate to severe plaque psoriasis in 2 Phase 3 studies. The pooled data from these studies showed improvements in the signs and symptoms at week 4.3 |
Baseline
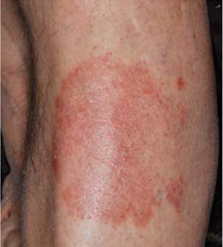
Week 2
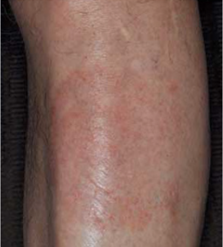
Week 4
Male, 84, with plaque psoriasis on his lower leg, treated with TOPICORT Spray, 0.25% twice daily for 4 weeks†
†Patient pictured was not a participant in the Phase 3 clinical studies for TOPICORT Spray. Individual results may vary.
• |
TOPICORT (desoximetasone) 0.25% Topical Spray is a corticosteroid indicated for the treatment of plaque psoriasis in patients 18 years of age or older. |
• |
TOPICORT Topical Spray is a topical corticosteroid that has been shown to suppress the hypothalamic-pituitary-adrenal (HPA) axis. |
• |
Systemic absorption of topical corticosteroids can produce reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of the topical corticosteroid. |
• |
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. |
• |
Local adverse reactions may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Reactions may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local reactions may be irreversible. |
• |
Safety and effectiveness of TOPICORT Topical Spray in patients younger than 18 years of age have not been studied; therefore use in pediatric patients is not recommended. |
References: 1. National Psoriasis Foundation. Topical steroids potency chart. https://www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart. Updated 2016. Accessed March 28, 2018. 2. TOPICORT Topical Spray [prescribing information]. Hawthorne, NY: Taro Pharmaceuticals U.S.A., Inc.; 2015. 3. Data on file. Hawthorne, NY: Taro Pharmaceuticals U.S.A., Inc. 4. Scheman A, Jacob S, Zirwas M, et al. Contact allergy: Alternatives for the 2007 North American Contact Dermatitis Group (NACDG) standard screening tray. Dis Mon. 2008;54(1-2):7-156. 5. Davis MDP, el-Azhary RA, Farmer SA. Results of patch testing to a corticosteroid series: a retrospective review of 1188 patients during 6 years at Mayo Clinic. J Am Acad Dermatol. 2007;56(6):921-927. 6. Scheuer E, Warshaw E. Allergy to corticosteroids: Update and review of epidemiology, clinical characteristics, and structural cross-reactivity. Am J Con Derm. 2003;14(4):179-187.