
HALOG (halcinonide, USP) 0.1% Cream or Ointment is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
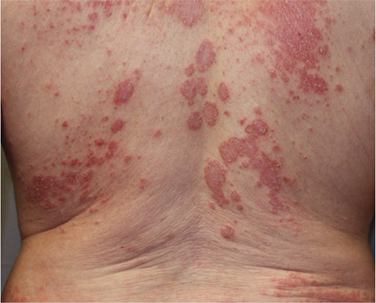
Baseline
Week 4
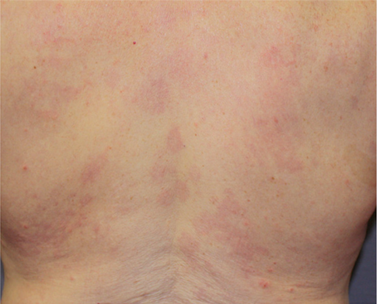
Male with eczema on the back, treated with HALOG Cream twice daily for 28 days*
*Individual patient results. Actual results may vary.
• |
HALOG (Halcinonide, USP) Cream or Ointment 0.1% is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. |
• |
Contraindications: Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparations. |
• |
Precautions: Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients. |
• |
Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. |
• |
Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children. |
• |
This medication is to be used as directed by the physician. It is for dermatologic use only. Avoid contact with the eyes. |
• |
Patients should be advised not to use this medication for any disorder other than for which it was prescribed. |
• |
The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive unless directed by the physician. |
• |
Patients should report any signs of local adverse reactions especially under occlusive dressing. |
• |
Parents of pediatric patients should be advised not to use tight-fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings. |
• |
Topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time. |
• |
Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman. |
• |
Adverse Reactions: The following local adverse reactions are reported infrequently with topical corticosteroids but may occur more frequently with the use of occlusive dressings (reactions are listed in an approximate decreasing order of occurrence): burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria. |
• |
This preparation is not for ophthalmic, oral, or intravaginal use. |
• |
For topical use only. |
References: 1. National Psoriasis Foundation. Topical steroid potency chart. https://www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart. Accessed February 4, 2019. 2. HALOG® Cream and Ointment [prescribing information]. Cranbury NJ: Sun Pharmaceutical Industries, Inc.; 2018. 3. Robinson RCV. Plastibase, a hydrocarbon gel ointment base. Bull Sch Med Univ Md. 1955;40(3):86-89. 4. Greenberg RG, Fowler J Jr. Biphasic release halcinonide provides immediate and sustained class 2 topical corticosteroid activity. Poster presented at: Winter Clinical Dermatology Meeting; January 14-29, 2012; Lahaina, HI. 5. Draelos ZD. Stratum corneum absorption kinetics of 2 potent topical corticosteroid formulations: a pilot study. Cutis. 2015;96(2):135-141.