|
|
|
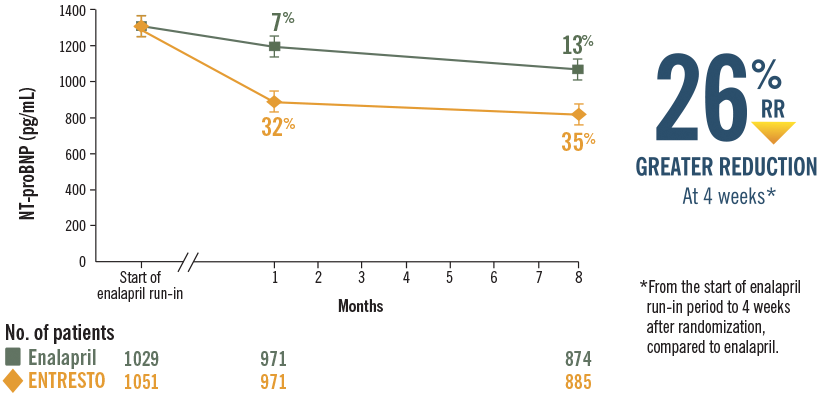
In PARADIGM-HF, ENTRESTO delivered rapid and sustained reductions in NT-proBNP vs enalapril7,8
|
|
Reductions in NT-proBNP were sustained through 8 months with ENTRESTO in a post hoc analysis8
|
|
|
ENTRESTO CV effects are attributed to increased levels of peptides and decreased angiotensin II effects, which resulted in decreased NT-proBNP9
|
|
|
|
ACEi=angiotensin-converting enzyme inhibitor; ANCOVA=analysis of covariance; ARB=angiotensin receptor blocker; CV=cardiovascular; E/e′=filling pressure (early diastolic filling velocity/early diastolic mitral annular velocity); HF=heart failure; HFrEF=heart failure with reduced ejection fraction; LAVI=left atrial volume index; LVEDVI=left ventricular end-diastolic volume index; LVEF=left ventricular ejection fraction; LVESVI=left ventricular end-systolic volume index; NT-proBNP=N-terminal pro–b-type natriuretic peptide; NYHA=New York Heart Association; RR=relative reduction.
|
|
Study Designs and Limitations, and Statistical Analyses for ENTRESTO
|
|
PARADIGM-HF STUDY DESIGN
|
|
PARADIGM-HF was a multinational, randomized, double-blind trial comparing ENTRESTO to enalapril in 8442 symptomatic (NYHA Class II–IV) adult systolic HF patients (LVEF ≤40%). After discontinuing their existing ACEi or ARB therapy, patients entered sequential single-blind run-in periods during which they received enalapril 10 mg BID, followed by ENTRESTO 100 mg (49/51 mg) BID, increasing to 200 mg (97/103 mg) BID. Patients who successfully completed the run-in periods were then randomized to receive either ENTRESTO 200 mg (97/103 mg) (n=4209) BID or enalapril 10 mg (n=4233) BID. The median follow-up duration was 27 months, and patients were treated for up to 4.3 years. For the primary end point, composite of CV death or first HF hospitalization, ENTRESTO was superior to enalapril (P<0.0001).9
|
|
PARADIGM-HF: POST HOC NT-proBNP ANALYSIS STUDY LIMITATIONS8,10
|
|
•
|
Biomarker measurements including NT‑proBNP, were drawn and analyzed in a sub‑group of PARADIGM‑HF patient population and therefore might not represent the entire cohort of systolic HF patients studied
|
|
•
|
Biological variability may influence the accuracy of a predictive value of a change in biomarkers. The change from baseline data should be interpreted in light of this influence
|
|
|
PROVE-HF STUDY DESIGN1,11
|
|
PROVE‑HF was a 52‑week, single‑arm, prospective, open‑label phase IV evaluation of 794 HFrEF patients in the United States. The primary end point was correlation of change in NT‑proBNP to change in cardiac remodeling parameters. At each study visit, a blood sample was sent to a central laboratory for measurement of plasma NT‑proBNP. Following completion, echocardiograms were transmitted in a secure fashion to a core laboratory, where they were interpreted following completion of all study protocols in a temporally and clinically blinded fashion.
|
|
PROVE-HF STUDY LIMITATIONS1
|
|
•
|
Observational, single-group, open-label design
|
|
•
|
A broad range of factors may affect NT-proBNP concentrations besides cardiac remodeling
|
|
•
|
Multiple comparisons may have increased risk of type 1 error
|
|
•
|
Not all echocardiographic measurements were available at each time point
|
|
•
|
Race was investigator-determined, with potential risk of inaccuracy
|
|
|
Please see full Prescribing Information, including Boxed
WARNING.
|
|
|
|
|
INDICATION
|
|
ENTRESTO is indicated to reduce the risk of cardiovascular death and
hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and
reduced ejection fraction.
|
|
ENTRESTO is usually administered in conjunction with other heart failure therapies, in place of
an ACE inhibitor or other ARB.
|
|
IMPORTANT SAFETY INFORMATION
|
|
WARNING: FETAL TOXICITY
|
|
•
|
When pregnancy is detected, discontinue ENTRESTO as soon as possible
|
|
•
|
Drugs that act directly on the renin‑angiotensin system can cause injury and death to the developing fetus
|
|
|
|
ENTRESTO is contraindicated in patients with hypersensitivity to any component. ENTRESTO is
contraindicated in patients with a history of angioedema related to previous
angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB)
therapy.
|
|
ENTRESTO is contraindicated with concomitant use of ACE inhibitors. Do not administer within 36
hours of switching from or to an ACE inhibitor. ENTRESTO is contraindicated with concomitant use
of aliskiren in patients with diabetes.
|
|
Angioedema: ENTRESTO may cause angioedema. Angioedema associated with laryngeal edema may
be fatal. ENTRESTO has been associated with a higher rate of angioedema in Black patients and in
patients with a prior history of angioedema. ENTRESTO should not be used in patients with
hereditary angioedema. If angioedema occurs, discontinue ENTRESTO immediately, provide
appropriate therapy, and monitor for airway compromise. ENTRESTO must not be re-administered.
|
|
Hypotension: ENTRESTO lowers blood pressure and may cause symptomatic hypotension.
Patients with an activated renin-angiotensin system, such as volume- and/or salt‑depleted
patients (e.g., those being treated with high doses of diuretics), are at greater risk. Correct
volume or salt depletion prior to administration of ENTRESTO or start at a lower dose. If
hypotension persists despite dose adjustment of diuretics, concomitant antihypertensive drugs,
and treatment of other causes of hypotension (e.g., hypovolemia) reduce the dosage or
temporarily discontinue ENTRESTO. Permanent discontinuation of therapy is usually not required.
|
|
Impaired Renal Function: Decreases in renal function may be anticipated in susceptible
individuals treated with ENTRESTO. In patients whose renal function depends upon the activity of
the renin‑angiotensin‑aldosterone system (e.g., patients with severe congestive
heart failure), treatment with ACE inhibitors and angiotensin receptor antagonists has been
associated with oliguria, progressive azotemia and, rarely, acute renal failure and death.
Closely monitor serum creatinine, and down‑titrate or interrupt ENTRESTO in patients who
develop a clinically significant decrease in renal function.
|
|
ENTRESTO may increase blood urea and serum creatinine levels in patients with bilateral or
unilateral renal artery stenosis. In patients with renal artery stenosis, monitor renal
function. Avoid use with aliskiren in patients with renal impairment (eGFR <60 mL/min/1.73
m2).
|
|
In patients who are elderly, volume‑depleted (including those on diuretic therapy), or
with compromised renal function, concomitant use of non‑steroidal anti‑inflammatory
drugs (NSAIDs), including COX‑2 inhibitors, with ENTRESTO may result in worsening of renal
function, including possible acute renal failure. These effects are usually reversible. Monitor
renal function periodically.
|
|
Hyperkalemia: Hyperkalemia may occur with ENTRESTO. Monitor serum potassium periodically
and treat appropriately, especially in patients with risk factors for hyperkalemia such as
severe renal impairment, diabetes, hypoaldosteronism, or a high potassium diet. Dosage reduction
or interruption of ENTRESTO may be required.
|
|
Concomitant use of potassium‑sparing diuretics (e.g., spironolactone, triamterene,
amiloride), potassium supplements, or salt substitutes containing potassium may lead to
increases in serum potassium.
|
|
ARBs: Avoid use of ENTRESTO with an ARB, because ENTRESTO contains the angiotensin II
receptor blocker valsartan.
|
|
Lithium: Increases in serum lithium concentrations and lithium toxicity have been
reported during concomitant administration of lithium with angiotensin II receptor antagonists.
Monitor serum lithium levels during concomitant use with ENTRESTO.
|
|
Common Adverse Events: In a clinical trial, the most commonly observed adverse events
with ENTRESTO vs enalapril, occurring at a frequency of at least 5% in either group, were
hypotension (18%, 12%), hyperkalemia (12%, 14%), cough (9%, 13%) dizziness (6%, 5%) and renal
failure/acute renal failure (5%, 5%).
|
|
Please see full Prescribing Information, including Boxed
WARNING.
|
|
|
|
|
|
References: 1. Januzzi JL Jr, Prescott MF, Butler J, et al; for the PROVE-HF Investigators. Association of change in N-terminal pro–b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction [published online ahead of print September 2, 2019]. JAMA. doi:10.1001/JAMA.2019.12821.
2. Daubert MA, Adams K, Yow E, et al. NT-proBNP goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF. JACC Heart Fail. 2019;7(2):158-168. doi:10.1016/j.jchf.2018.10.014.
3. Weiner RB, Baggish AL, Chen-Tournoux A, et al. Improvement in structural and functional echocardiographic parameters during chronic heart failure therapy guided by natriuretic peptides: mechanistic insights from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) study. Eur J Heart Fail. 2013;15(3):342-351. doi:10.1093/eurjhf/hfs180.
4. Cohn JN, Ferrari R, Sharpe N; on behalf of an International Forum on Cardiac Remodeling. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35(3):569-582.
5. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4(1):98-108.
6. Udelson JE, Konstam MA. Ventricular remodeling: fundamental to the progression (and regression) of heart failure. J Am Coll Cardiol. 2011;57(13):1477-1479.
7. Velazquez EJ, Morrow DA, DeVore AD, et al; for the PIONEER-HF Investigators. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;383(6):539-548.
8. Zile MR, Claggett BL, Prescott MF, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68(22):2425-2436.
9. ENTRESTO [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; October 2019.
10. McMurray JJV, Packer M, Desai AS, et al; for the PARADIGM-HF Investigators and Committees. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
11. Januzzi JL Jr, Prescott MF, Butler J, et al; for the PROVE-HF Investigators. Association of change in N-terminal pro–b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction [published online ahead of print September 2, 2019]. JAMA. doi:10.1001/JAMA.2019.12821.
Supplementary material accessed at https://jamanetwork.com/journals/jama/article-abstract/2749476?utm_campaign=articlePDF%26utm
_medium%3darticlePDFlink%26utm_source%3darticlePDF%26utm_content%3djama.2019.12821. Accessed
October 30, 2019.
|
|
If you wish to unsubscribe from future email communications about this program from Novartis Pharmaceuticals Corporation, please click here.
|
|
If you have any questions concerning email marketing or marketing communications from Novartis Pharmaceuticals Corporation, please call 1‑888‑NOW‑NOVA (1‑888‑669‑6682), Monday-Friday, 8:30 AM-5:00 PM ET.
|
|
For more information, please visit the Privacy Policy.
|
|
ENTRESTO and the ENTRESTO logo are registered trademarks of Novartis AG.
|
|
| |
|
Novartis Pharmaceuticals Corporation
One Health Plaza
East Hanover, New Jersey 07936-1080
|
|
|